Path ID: DB01142_MESH_D009450_1
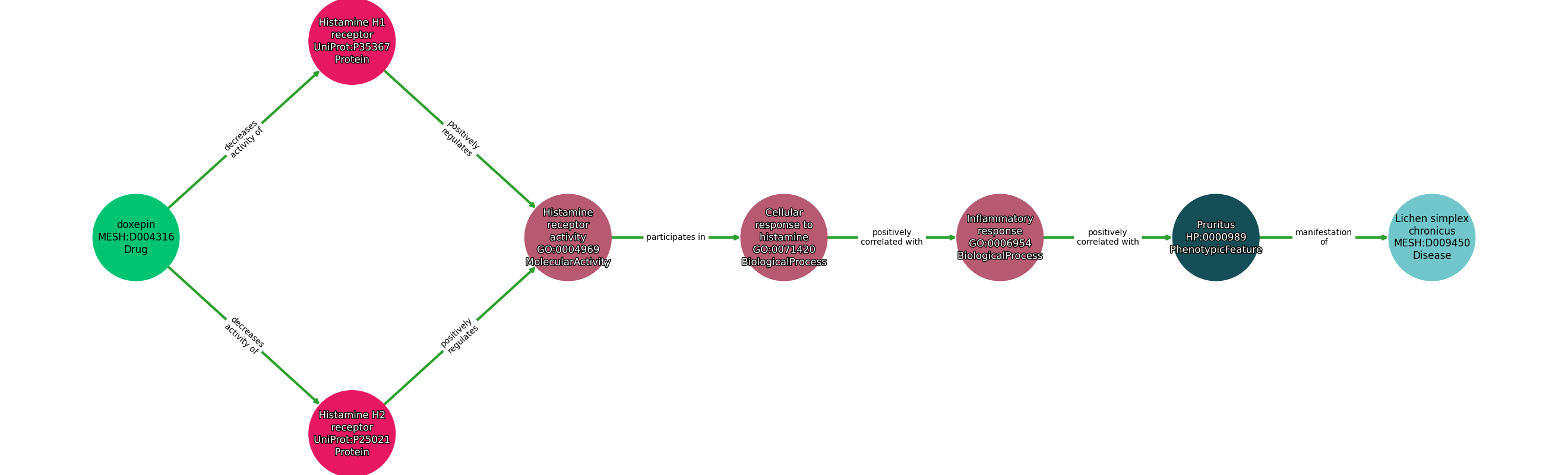
Concepts
| Identifier | Name | Type |
|---|---|---|
| MESH:D004316 | doxepin | Drug |
| UniProt:P35367 | Histamine H1 receptor | Protein |
| UniProt:P25021 | Histamine H2 receptor | Protein |
| GO:0004969 | Histamine receptor activity | MolecularActivity |
| GO:0071420 | Cellular response to histamine | BiologicalProcess |
| GO:0006954 | Inflammatory response | BiologicalProcess |
| HP:0000989 | Pruritus | PhenotypicFeature |
| MESH:D009450 | Lichen simplex chronicus | Disease |
Relationships
NOTE: predicates are annotated in Biolink Model (v1.3.0)
| Subject | Predicate | Object |
|---|---|---|
| Doxepin | DECREASES ACTIVITY OF | Histamine H1 Receptor |
| Doxepin | DECREASES ACTIVITY OF | Histamine H2 Receptor |
| Histamine H1 Receptor | POSITIVELY REGULATES | Histamine Receptor Activity |
| Histamine H2 Receptor | POSITIVELY REGULATES | Histamine Receptor Activity |
| Histamine Receptor Activity | PARTICIPATES IN | Cellular Response To Histamine |
| Cellular Response To Histamine | POSITIVELY CORRELATED WITH | Inflammatory Response |
| Inflammatory Response | POSITIVELY CORRELATED WITH | Pruritus |
| Pruritus | MANIFESTATION OF | Lichen Simplex Chronicus |
Comment: Topical doxepin is approved for short-term (up to 8 days) management of moderate pruritus in adult patients with atopic dermatitis, pruritus or lichen simplex chronicus. Antihistamine properties of doxepin is beleived to be MoA
Reference: